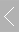电池原位红外附件

电化学原位红外光谱分析是红外分析技术的一个重要分支,能够定性分析电催化(如CO2电还原等)反应、各种类型电池(如锂离子、锂硫电池等)充放电过程中电极表面的产物或中间产物随时间(电位)不断变化的趋势,是研究电化学反应机理以及电化学反应动力学的重要手段之一。
构造原理:
(1)两电极体系,专为电池体系设计。
(2)电化学反应池气密性良好,可通入反应气体。
(3)金刚石晶体,适用性广。
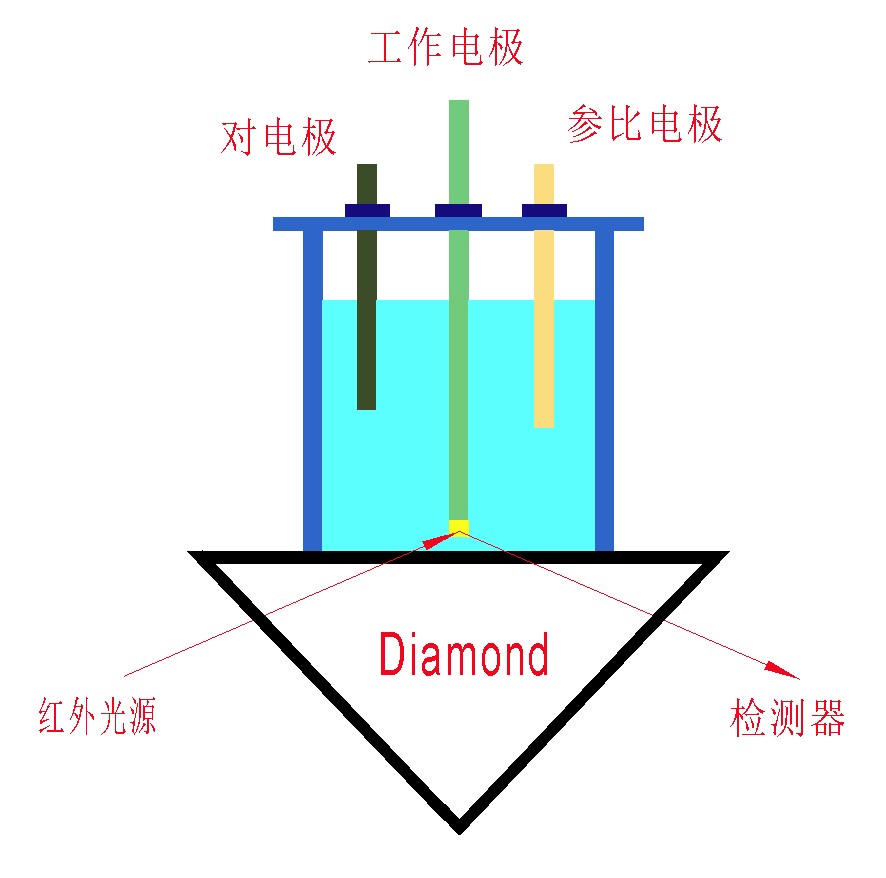
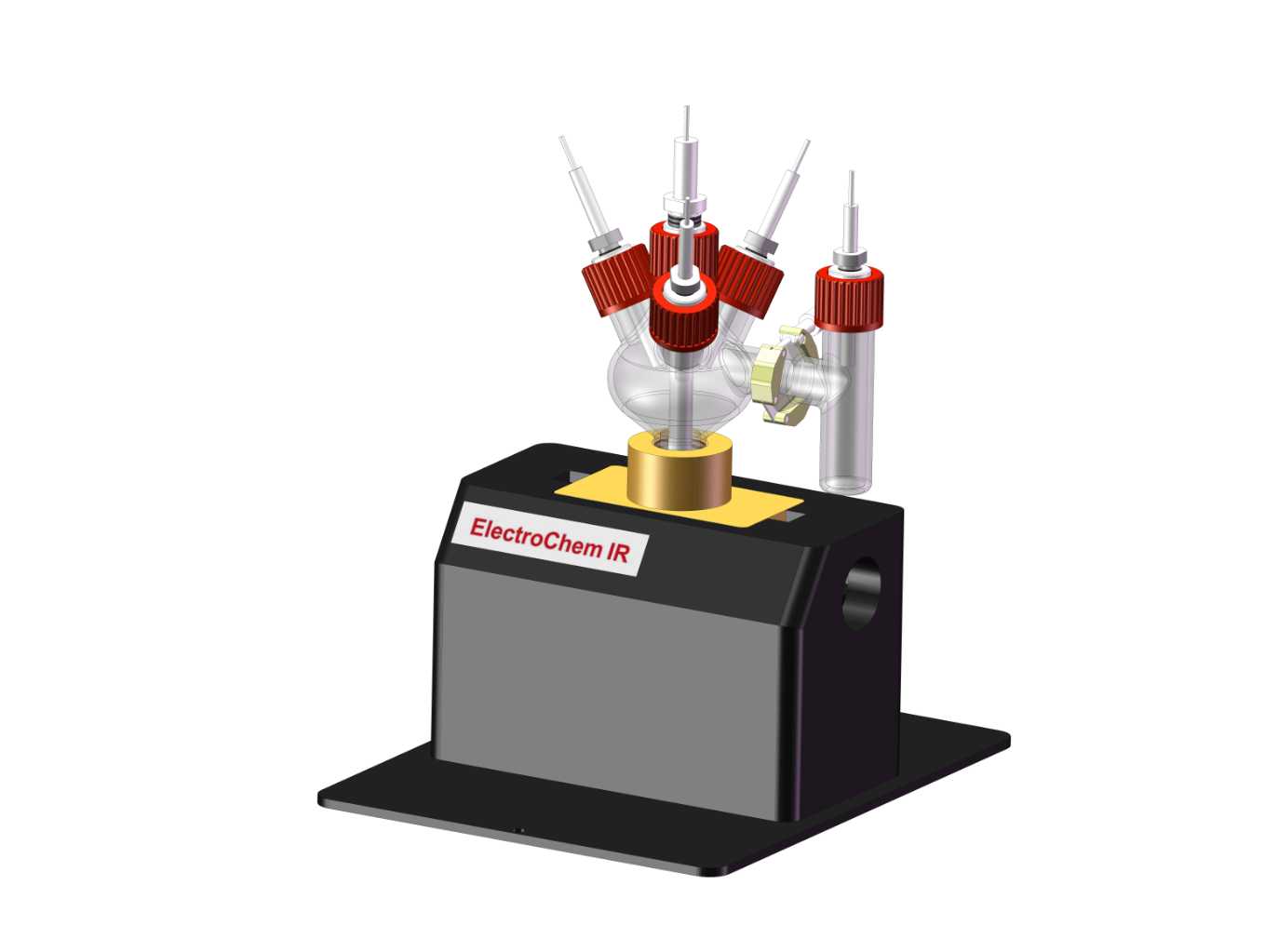
图2:基本原理示意图
附件组成
(1)红外光谱仪主机适配底板,适配主流红外光谱仪。
(2)光路系统。
(3)PEEK材质气密性电化学池。
(4)O型圈密封件。
主要特点
(1)优化的光路系统,光通量大。
(2)电化学池密封性能好,可通入反应气体。
(3)金刚石晶体光通量大。
(4)独特的电极,电解液信号采集调节技术。
(5)可实现电化学红外质谱三联用。
(6)金刚石晶体板和电化学池拆卸方便,可方便在手套箱中组装电池。
(7)提供现场技术服务。
主要技术参数
1.光谱范围:250/525-4000 cm-1
2.晶体种类:金刚石晶体
3.电化学池:PEEK材质,两电极体系,气密性池体,可方便在手套箱中装卸电池,设有进气口和出气口,可实现各类电池充放电过程中红外光谱的采集。
4.温控电化学池,温控范围:RT-100℃,温控精度0.1℃。
5.电极与金刚石晶体距离调节系统,带刻度微调功能,重现性好,以实现观测电解液溶剂化或电极表面物种变化。
6.电化学池可实现电化学质谱仪与红外三联用,提供多联用技术方案。
7.反射次数:单次反射。
8.反射类型:外反射。
9.光路反射系统适配主流品牌红外光谱仪,提供光谱仪适配底板,光路系统方便安放或取出光谱仪样品仓。
应用案例
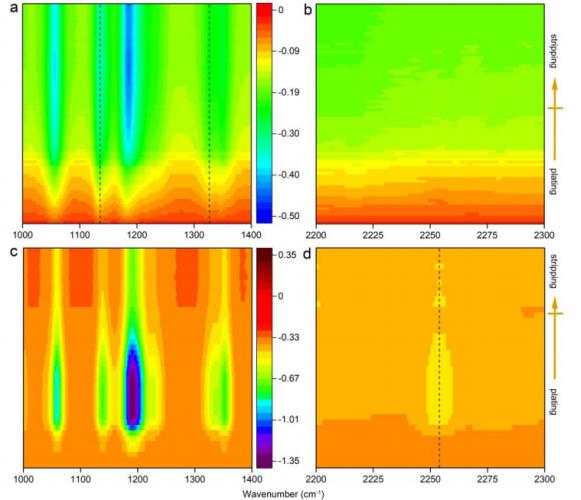
锂离子电池 Chem. Mater. 2020, 32, 8, 3405–3413
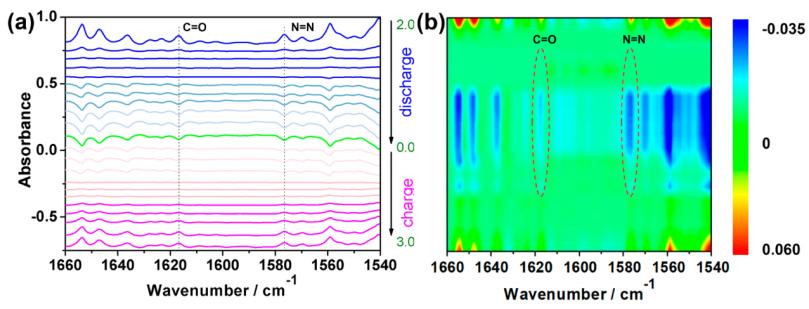
锂离子电池 ACS Energy Lett. 2020, 5, 1022−1031
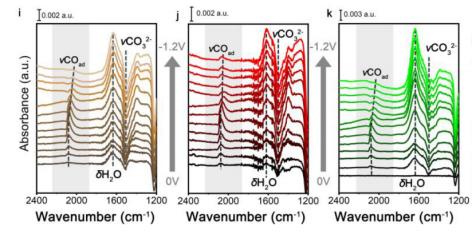
锌离子电池 Adv. Funct. Mater. 2020, 2003890
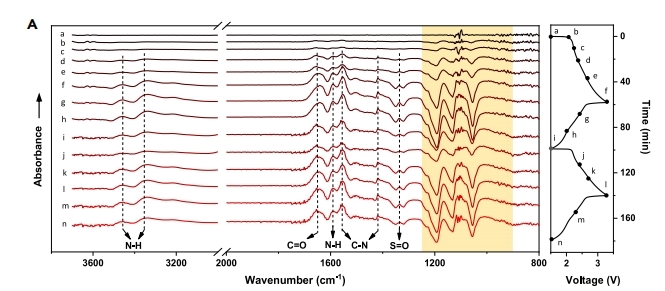
锂离子电池 Joule 2022, 6, 399–417
部分客户论文发表清单:
Jianping Xiao*, Bin Zhang*, et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nat. Commun. 2020, 11, 3415
Lei Yan, Yonggang Wang*, et al. Chemically Self-Charging Aqueous Zinc-Organic Battery. J. Am. Chem. Soc. 2021, 143, 15369-15377
Bingliang Wang, Yongyao Xia*, et al. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 2020, 13, 2200-2208
Yang Peng*, et al. Breaking Linear Scaling Relationship by Compositional and Structural Crafting of Ternary Cu-Au/Ag Nanoframes for Electrocatalytic Ethylene Production. Angew. Chem. Int. Ed. 2021, 60, 2508-2518
Zhuo Yu, Yonggang Wang*, et al. Boosting Polysulfide Redox Kinetics by Graphene-Supported Ni Nanoparticles with Carbon Coating. Adv. Energy Mater. 2020, 10, 2000907
Xinwei Ding, Zhi Yang*, et al. Biomimetic Molecule Catalysts to Promote the Conversion of Polysulfides for Advanced Lithium–Sulfur Batteries Adv. Funct. Mater. 2020, 30, 2003354
Hong Guo*, Xueliang Sun*, et al. Dual Active Site of the Azo and Carbonyl-Modified Covalent Organic Framework for High-Performance Li Storage. ACS Energy Lett. 2020, 5, 1022-1031
Bin Zhang* et al. Superficial Hydroxyl and Amino Groups Synergistically Active Polymeric Carbon Nitride for CO2 Electroreduction. ACS Catal. 2019, 9, 10983-10989
Suya Zhou, Zhi Yang*, et al. Dual-Regulation Strategy to Improve Anchoring and Conversion of Polysulfides in Lithium–Sulfur Batteries ACS Nano. 2020, 14, 7538–7551
Yongyao Xia*, et al. Low-Temperature Charge/Discharge of Rechargeable Battery Realized by Intercalation Pseudocapacitive Behavior. Adv. Sci. 2020, 7, 2000196
Lei Wang*, Yonggang Wang, et al. Pencil-drawing on nitrogen and sulfur co-doped carbon paper: An effective and stable host to pre-store Li for high-performance lithium–air batteries. Energy Storage Materials. 2020, 26, 593-603
Bin Zhang, et al. Unveiling in situ evolved In/In2O3− x heterostructure as the active phase of In2O3 toward efficient electroreduction of CO2 to formate. Science Bulletin. 2020, 65, 1547-1554
Huani Li, Shubiao Xia*, Hong Guo*, et al. Red Phosphorus Confined in Hierarchical Hollow Surface-Modified Co9S8 for Enhanced Sodium Storage. Sustainable Energy Fuels. 2020, 4, 2208-2219
Guanglei Cui*, Liquan Chen, et al. Non-flammable nitrile deep eutectic electrolyte enables high voltage lithium metal batteries. Chem. Mater. 2020, 32, 3405-3413
Guanglei Cui*, et al. Investigation on the Cathodic Interfacial Stability of Nitrile Electrolyte and its performance with High Voltage LiCoO2 Chem. Commun. 2020, 56, 4998-5001
Zhongbin Zhuang*, et al. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 2020, 11, 5651
Tiancun Liu, Yong Wang*, et al. Organic supramolecular protective layer with rearranged and defensive Li deposition for stable and dendrite-free lithium metal anode. Energy Storage Materials. 2020, 32, 261–271
X. Yin, Y. Wang*, et al. Designing cobalt-based coordination polymers for high-performance sodium and lithium storage: from controllable synthesis to mechanism detection. Materials Today Energy. 2020, 17, 100478
Song Chen, Jintao Zhang*, et al. Regulation of Lamellar Structure of Vanadium Oxide via Polyaniline Intercalation for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2020, 30, 2003890
Yanrong Xue, Zhongbin Zhuang*, et al. Sulfate-Functionalized RuFeOx as Highly Efficient Oxygen Evolution Reaction Electrocatalyst in Acid. Adv. Funct. Mater. 2021, 31, 2101405
Hong Guo*, et al. Cooperative catalytic interface accelerates redox kinetics of sulfur species for high-performance Li-S batteries. Energy Storage Materials. 2021, 40, 139-149
Bin Zhang*, et al. Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping. SCIENCE CHINA Chemistry. 2021, 64, 1493–1497
Yang Peng*, et al. Geometric Modulation of Local CO Flux in Ag@Cu2O Nanoreactors for Steering the CO2RR pathway toward High-Efficacy Methane Production. Adv. Mater. 2021, 33, 2101741
Yonggang Wang*, et al. Molecular Tailoring of n/p-type Phenothiazine Organic Scaffold for Zinc Batteries. Angew. Chem. Int. Ed. 2021, 60, 20826-20832
Hongliang Jiang*, Chunzhong Li*, et al. Dynamically Formed Surfactant Assembly at the Electrified Electrode–Electrolyte Interface Boosting CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 6613–6622
Yang Peng*, et al. Au-activated N motifs in non-coherent cupric porphyrin metal organic frameworks for promoting and stabilizing ethylene production. Nat. Commun. 2022, 13, 63
Jie Zeng*, et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nature Nanotechnology. 2021, 16, 1386-1393
Min-Rui Gao*, et al. Identification of Cu(100)/Cu(111) Interfaces as Superior Active Sites for CO Dimerization During CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 1, 259-269
Chen Feng, Shiming Zhou*, Jie Zeng*, et al. Tuning the Electronic and Steric Interaction at the Atomic Interface for Enhanced Oxygen Evolution. J. Am. Chem. Soc. 2022, 144,21,9271-9279
Rui Lin, Jianhui Wang, et al. Asymmetric donor-acceptor moleculeregulated core-shell-solvation electrolyte for high-voltage aqueous batteries. Joule 2022, 6, 399–417
Xiaogang Zhang*, et al. Successive Cationic and Anionic (De)-Intercalation/Incorporation into an Ion-Doped Radical Conducting Polymer. Batteries & Supercaps 2019, 2, 979-984
Zhongju Wang, Yongzhu Fu*, et al. Biredox‐Ionic Anthraquinone‐Coupled Ethylviologen Composite Enables Reversible Multielectron Redox Chemistry for Li‐Organic Batteries. Adv. Sci. 2022, 9, 2103632
Jintao Zhang*, et al. Defect evolution of hierarchical SnO2 aggregatesfor boosting CO2 electrocatalytic reduction. J. Mater. Chem. A 2021, 9, 14741-14751
Fei Ai, Yijun Lu*, et al. Heteropoly acid negolytes for high-power-density aqueous redox flow batteries at low temperatures. Nature Energy 2022, 7, 417–426
Zhejun Li, Yijun Lu*. Polysulfide-based redox flow batteries with long life and low levelized cost enabled by charge-reinforced ion-selective membranes. Nature Energy 2021, 6, 517–528
Shanshan Lu, Wei Zhou. et al. Phenanthrenequinone-like moiety functionalized carbon for electrocatalytic acidic oxygen evolution. Chem. 2022, 8, 1415-1426.
Tieliang Li, Yifu Yu, Bin Zhang*, et al. Sulfate-Enabled Nitrate Synthesis from Nitrogen Electrooxidation on Rhodium Electrocatalyst. Angew. Chem. Int. Ed. 2022, e202204541
Yanbo Li, Bin Zhang, Yifu Yu*, et al. Electrocatalytic Reduction of Low-Concentration Nitric Oxide into Ammonia over Ru Nanosheets. ACS Energy Letters 2022, 7, 1187-1194
Yanmei Huang, Yifu Yu, Bin Zhang*, et al. Direct Electrosynthesis of Urea from Carbon Dioxide and Nitric Oxide. ACS Energy Letters 2022, 7, 284-291
Wenfu Xie, Hao Li, Min Wei*, et al. NiSn Atomic Pair on Integrated Electrode for Synergistic Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 7382–7388
Rui Sui, Jiajing Pei, Zhongbin Zhuang*, et al. Engineering Ag−Nx Single-Atom Sites on Porous Concave N-Doped Carbon for Boosting CO2 Electroreduction. ACS Appl. Mater. Interfaces 2021, 13, 17736-17744
Tiliang Li, Yuting Wang, Yifu Yu*, Bin Zhang*, et al. Ru-Doped Pd Nanoparticles for Nitrogen Electrooxidation to Nitrate. ACS Catal. 2021, 11, 14032-14037
Bin Zhang*, et al. Promoting selective electroreduction of nitrates to ammonia over electron-deficient Co modulated by rectifying Schottky contacts. Science China Chemistry 2020, 63, 1469-1476
Jiangwei Shi, Bin Zhang*, et al. Promoting nitric oxide electroreduction to ammonia over electron-rich Cu modulated by Ru doping. Science China Chemistry 2021, 64, 1493-1497
Jintao Zhang* et al. Atomic Bridging Structure of Nickel-Nitrogen-Carbon for Highly Efficient Electrocatalytic Reduction of CO2. Angew. Chem.Int. Ed. 2022, 61, e202113918
Lang Xu* et al. Gadolinium Changes the Local Electron Densities of Nickel 3d Orbitals for Efficient Electrocatalytic CO2 Reduction. Angew. Chem.Int. Ed. 2022, 61, e202201166
Bin Zhang* et al. Phenanthrenequinone-like moiety functionalized carbon for electrocatalytic acidic oxygen evolution. Chem. 2022, 8, 1415-1426
Sheng Dai*, Minghui Zhua*, Yifan Han* et al. Probing the role of surface hydroxyls for Bi, Sn and In catalysts during CO2 Reduction. Applied Catalysis B: Environmental 2021, 298,
Nan Wang, Yonggang Wang*, et al. Zinc-organic Battery with a Wide Operation-temperature Window from -70 to 150 oC. Angew. Chem. Int. Ed. 2020,59,14577-14583
Nannan Meng, Yifu Yu, Bin Zhang*, et al. Efficient Electrosynthesis of Syngas with Tunable CO/H2 Ratios over ZnxCd1-xS-Amine Inorganic-Organic Hybrids. Angew. Chem. Int. Ed. 2019, 58, 18908–18912